
Ĭonverting Weight Fraction to Mole Fraction In General For a binary mixture: For a mixture of C components: Įnthalpy vs. Although slight, one can begin to see the effect of pressure on the azeotropic point. Weight Fraction Converting from wt fraction of the azeotrope to mole fraction: Thus, the azeotropic mole fraction is greater at P = 1 Kg/cm 2 than at 1 atm: 0.902 vs. For ethanol-water, this can be readily done using the molecular weights, MW EtOH =46.07 and MW w = 18.02. Weight Fraction Note that the enthalpy- composition plot is presented in terms of weight fractions – we will typically use mole fractions so one must convert between the two. Composition – Ponchon-Savarit Plot Note the boiling temperatures of the pure components, water and ethanol, and the temperature of the azeotrope are different due to the pressure at which the data was taken: P = 1 kg/cm 2 (0.97 atm) 1 atm Water 99.1 o C 100 o C Ethanol 77.8 78.30 Azeotrope 77.65 78.15 x,y plots for ethanol-water? Įnthalpy vs. Why is this different than that determined from the y vs. x,y plots for ethanol-water? The azeotrope for ethanol-water is indicated as T = 77.65 o C and a concentration of 0.955. Why? Why are the boiling point temperatures of the pure components different than those determined from the y vs. An azeotrope is indicated by the composition at which the isotherm becomes vertical. Points between the saturated liquid line and the saturated vapor line represent a two-phase, liquid-vapor system. Temperature is represented by isothermal tie lines between the saturated liquid (boiling) line and the saturated vapor (dew) line. Composition – Ponchon-Savarit Plot 3 phases are shown on the plot – solid, liquid, and vapor. Composition – Ponchon-Savarit PlotĮnthalpy vs. composition plot to obtain this information. We will also need to employ energy balances, based on enthalpy, for certain separation problems. Composition – Ponchon-Savarit Plot We have begun to employ mass balances, both total and component. Images are best displayed at 300x275 pixels.Enthalpy vs. csv file must be accurate for the icon to be loaded and displayed. It should be noted that the image path/filename given in the. If new images are added, they should be placed within the /img directory. The image value can left blank without consequence, and will just result in no image being displayed for that compound. (Please note the coefficeint examples ARE NOT actual values)
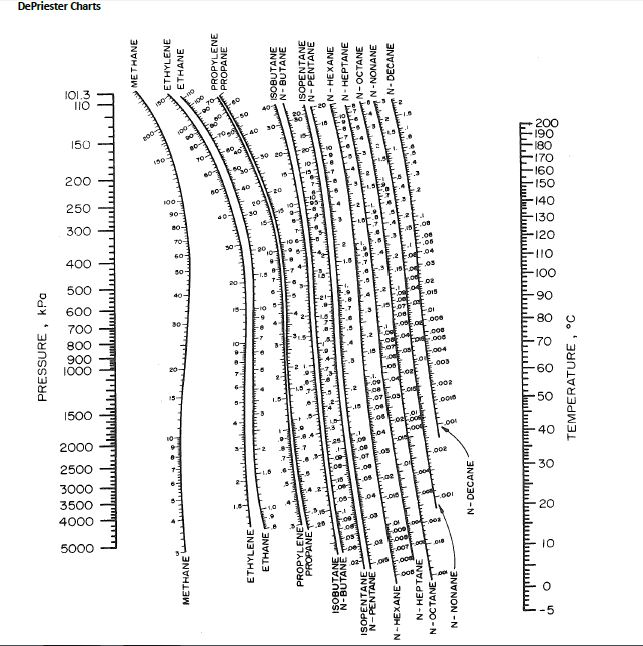
There are a total of 13 entries per compound in the. Each compound is located on a new line (row) in the. command: java -jar kcalc.jar path/m圜ompoundData.csv) Adding New CompoundsĪdding new compounds can be trivially done by editing the. csv file that is not the default, simply call the program from terminal using the. To run the program using compound information from a. jar file is located in, there must be a directory called img, in which all compound icons are located in. csv path and file name.Ībove the directory the. jar without arguments through the terminal, it will launch using the default. csv file, from which all the compound names, coefficients, and icons paths are. jar can be called from terminal using the standard command: java -jar kcalc.jar. jar can be run by double clicking on the icon from a file explorer. This calculator essentially acts as a DePrieseter chart, calculating the vapor-liquid equilibrium ratio (ie K-Value) for a variety of compounds at a particular temperature and pressure.


 0 kommentar(er)
0 kommentar(er)
